Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sato, Tetsuya; Nagame, Yuichiro*
Nihon Butsuri Gakkai-Shi, 78(2), p.64 - 72, 2023/02
The study of the chemistry of superheavy elements, which are located in the heavy extremes of the periodic table, has made considerable progress over the past 20 years, and new approaches based on various ideas have recently been developed. Research groups in Japan have also made significant contributions to the development of research on superheavy elements. Recently, notable results have been reported for the transactinide elements rutherfordium (element 104), dubnium (element 105), and seaborgium (element 106), and the heavy actinides with atomic numbers exceeding 100. The review will focus on the recent main results of these elements. This review outlines the main recent results and touches on future prospects.
G tz, M.*; Yakushev, A.*; G
tz, M.*; Yakushev, A.*; G tz, S.*; Di Nitto, A.*; D
tz, S.*; Di Nitto, A.*; D llmann, Ch. E.*; Asai, Masato; Kindler, B.*; Krier, J.*; Lommel, B.*; Nagame, Yuichiro*; et al.
llmann, Ch. E.*; Asai, Masato; Kindler, B.*; Krier, J.*; Lommel, B.*; Nagame, Yuichiro*; et al.
Radiochimica Acta, 110(2), p.75 - 86, 2022/02
Times Cited Count:2 Percentile:30.55(Chemistry, Inorganic & Nuclear)The study of volatile superheavy element carbonyl complexes requires more efficient methods because the yield of transactinide elements decreases with increasing atomic number. This is achieved by using a newly developed double chamber system to separate the recoil chamber and the reaction one, thereby avoiding the decomposition of reactive molecules by the projectile ion beam, which hinders the synthesis of carbonyl complexes. The feasibility of this method was verified by synthesizing 5d metal short-lived isotopes as homologous element isotopes of the light transactinide elements Sg, Bh, Hs, and Mt at the Japan Atomic Energy Agency tandem accelerator and conducting model experiments.
Yakushev, A.*; Lens, L.*; D llmann, C. E.*; Block, M.*; Nagame, Yuichiro*; Sato, Tetsuya; Toyoshima, Atsushi*; 42 of others*
llmann, C. E.*; Block, M.*; Nagame, Yuichiro*; Sato, Tetsuya; Toyoshima, Atsushi*; 42 of others*
Frontiers in Chemistry (Internet), 9, p.753738_1 - 753738_9, 2021/11
Times Cited Count:11 Percentile:62.25(Chemistry, Multidisciplinary)Nihonium (Nh, element 113) and flerovium (Fl, element 114) are the first superheavy elements in which the 7p shell is occupied. High volatility and inertness were predicted for Fl due to the strong relativistic stabilization of the closed 7p sub-shell, which originates from a large spin-orbit splitting between the 7p
sub-shell, which originates from a large spin-orbit splitting between the 7p and 7p
and 7p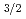 orbitals. One unpaired electron in the outermost 7p
orbitals. One unpaired electron in the outermost 7p sub-shell in Nh is expected to give rise to a higher chemical reactivity. Theoretical predictions of Nh reactivity are discussed, along with results of the first experimental attempts to study Nh chemistry in the gas phase. The experimental observations verify a higher chemical reactivity of Nh atoms compared to its neighbor Fl and call for the development of advanced setups. First tests of a newly developed detection device miniCOMPACT with highly reactive Fr isotopes assure that effective chemical studies of Nh are within reach.
sub-shell in Nh is expected to give rise to a higher chemical reactivity. Theoretical predictions of Nh reactivity are discussed, along with results of the first experimental attempts to study Nh chemistry in the gas phase. The experimental observations verify a higher chemical reactivity of Nh atoms compared to its neighbor Fl and call for the development of advanced setups. First tests of a newly developed detection device miniCOMPACT with highly reactive Fr isotopes assure that effective chemical studies of Nh are within reach.

Chiera, N. M.*; Sato, Tetsuya; Eichler, R.*; Tomitsuka, Tomohiro; Asai, Masato; Adachi, Sadia*; Dressler, R.*; Hirose, Kentaro; Inoue, Hiroki*; Ito, Yuta; et al.
Angewandte Chemie; International Edition, 60(33), p.17871 - 17874, 2021/08
Times Cited Count:3 Percentile:14.19(Chemistry, Multidisciplinary)The formation and the chemical characterization of single atoms of dubnium (Db, element 105), in the form of its volatile oxychloride, was investigated using the on-line gas phase chromatography technique, in the temperature range 350 - 600  C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl
C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl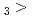 TaOCl
TaOCl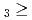 DbOCl
DbOCl . From the obtained experimental results, thermochemical data for DbOCl
. From the obtained experimental results, thermochemical data for DbOCl were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Ito, Yuta; Shirai, Kaori*; Suzuki, Hayato; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; et al.
Journal of Radioanalytical and Nuclear Chemistry, 320(3), p.633 - 642, 2019/06
Times Cited Count:2 Percentile:10.81(Chemistry, Analytical)An isothermal gas-chromatographic (IGC) device has been developed and tested for on-line gas phase studies of volatile oxychlorides of short-lived group-5 transition metals. Radioisotopes of niobium and tantalum, produced in nuclear fusion evaporation reactions, are directly flushed into the IGC setup by an inert gas-jet. Oxychloride compounds are formed by the addition of SOCl and O
and O . Parameters influencing the formation and transport of NbOCl
. Parameters influencing the formation and transport of NbOCl and TaOCl
and TaOCl are investigated. For nuclides with half-lives (
are investigated. For nuclides with half-lives (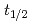 ) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of
) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of  Db(
Db(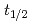 = 34s).
= 34s).
Nagame, Yuichiro
Radioisotopes, 54(11), p.555 - 567, 2005/11
no abstracts in English
Haba, Hiromitsu*; Nagame, Yuichiro
Gendai Kagaku, (405), p.32 - 38, 2004/12
no abstracts in English
Nakamura, Akio
Nihon Genshiryoku Gakkai-Shi, 45(11), p.736 - 737, 2003/11
no abstracts in English
Kaneko, Tetsuya*; Ono, Sawako*; Goto, Shinichi*; Haba, Hiromitsu; Asai, Masato; Tsukada, Kazuaki; Nagame, Yuichiro; Kudo, Hisaaki*
Journal of Radioanalytical and Nuclear Chemistry, 255(2), p.381 - 384, 2003/02
Times Cited Count:0 Percentile:0.01(Chemistry, Analytical)no abstracts in English
Sato, Tetsuya; Chiera, N. M.*; Tomitsuka, Tomohiro; Tokoi, Katsuyuki*; Suzuki, Hayato*; Ito, Yuta; Asai, Masato; Shirai, Kaori*; Inoue, Hiroki*; Adachi, Sadia*; et al.
no journal, ,
The influence of strong relativistic effects on chemical properties has been interesting in the superheavy element region. Their chemical properties, however, have not been investigated sufficiently because of experimental difficulties owing to their low production rates and short half-lives. In order to elucidate the chemical properties of dubnium (Db, Z = 105), we have conducted on-line isothermal gas chromatographic experiments of oxychloride of group-5 elements. We confirmed the formation of volatile oxychlorides of Db and its lighter homologs Nb and Ta by using  Db (half-life,
Db (half-life, 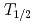 = 33.8 s),
= 33.8 s),  Nb (
Nb (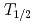 = 14.5 min.), and
= 14.5 min.), and 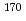 Ta (
Ta (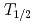 = 6.76 min.), respectively. We successfully determined the adsorption enthalpies of the oxychlorides of each element on the quartz surface from their isothermal gas chromatographic behavior. The obtained volatility sequence of the group-5 elements is found to be Nb
= 6.76 min.), respectively. We successfully determined the adsorption enthalpies of the oxychlorides of each element on the quartz surface from their isothermal gas chromatographic behavior. The obtained volatility sequence of the group-5 elements is found to be Nb  Ta
Ta  Db.
Db.
Natori, Hina; Sato, Tetsuya; Asai, Masato; Ito, Yuta; Uchibaba, Yuta; Gong, G.; Tsukada, Kazuaki; Miyachi, Yuta; Nagame, Yuichiro*
no journal, ,
To elucidate the chemical properties of element 106, seaborgium (Sg), we have constructed an off-line isothermal gas chromatographic system. As a model experiment for Sg using the system, gas-phase chemical experiments were performed for volatile oxychlorides of short-lived molybdenum (Mo) isotopes, a homolog of Sg, produced in spontaneous fission of 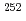 Cf. The optimum experimental conditions were obtained for isothermal gas chromatographic experiments using this apparatus. The observed isothermal gas chromatographic behaviors of short-lived Mo isotopes, we have successfully determined values of the adsorption enthalpies of Mo oxychlorides on the column surface. These values were in good agreement with those estimated from the sublimation enthalpies of the compounds.
Cf. The optimum experimental conditions were obtained for isothermal gas chromatographic experiments using this apparatus. The observed isothermal gas chromatographic behaviors of short-lived Mo isotopes, we have successfully determined values of the adsorption enthalpies of Mo oxychlorides on the column surface. These values were in good agreement with those estimated from the sublimation enthalpies of the compounds.